Science has given us the tools to stop viral hepatitis. Despite this, deaths due to hepatitis are on the rise.



THE REALITY
Every 30 seconds someone loses their life to viral hepatitis
It doesn’t have to be this way
The health and financial returns are clear. If we want to reach the UN Sustainable Development Goals, we must come together to change this reality.
OUR APPROACH
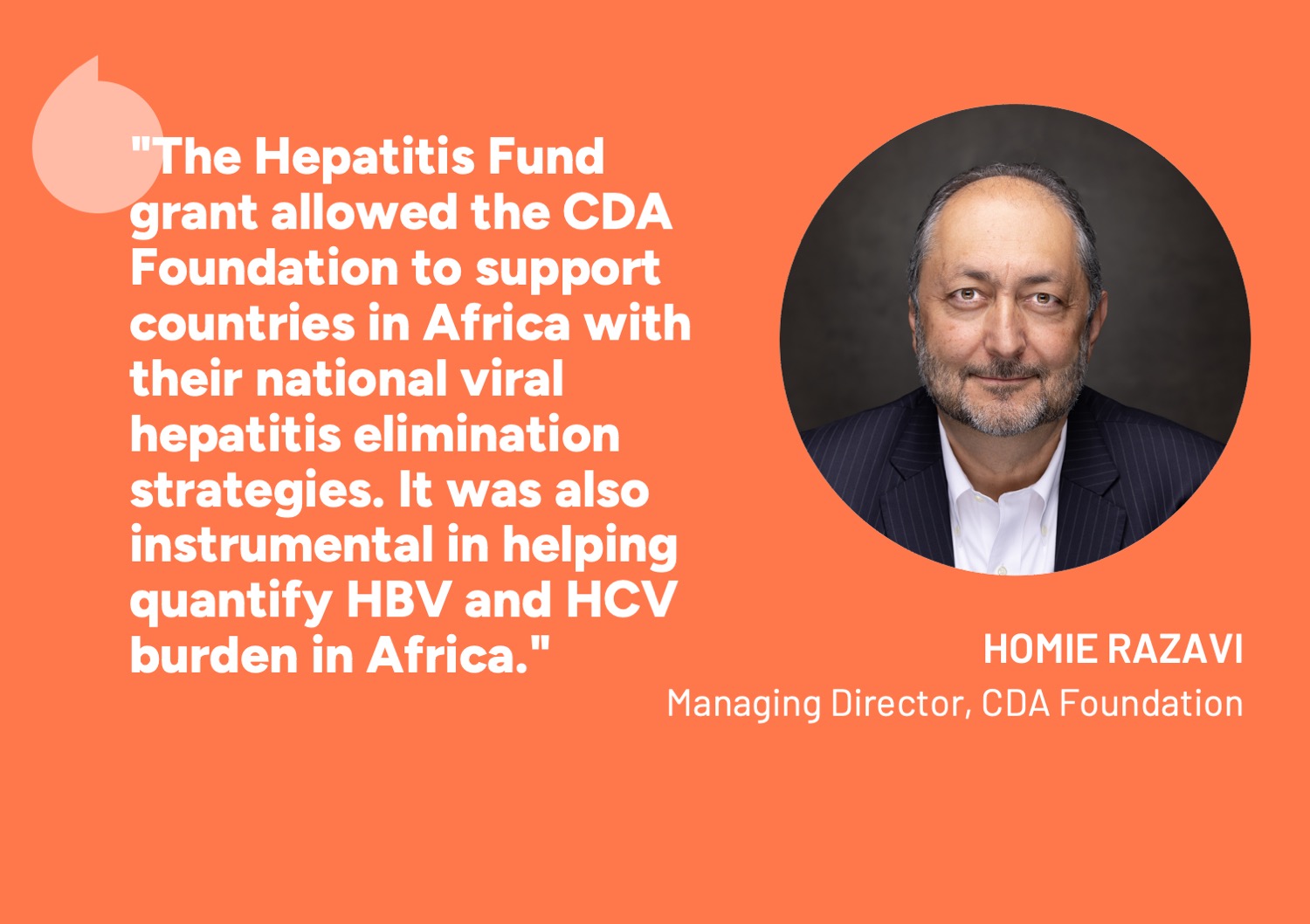
The Hepatitis Fund
We are a global funding platform dedicated to ending viral hepatitis. Making the most of the opportunities science presents, we invest in focused, high-impact projects that inspire national and global action.





The impact
We are the spark that sets change in motion
* Tested: 204,530 / Diagnosed: 30,360
"Every 30 sec someone dies of hepatitis related illness. Be part of a movement that changes that – help us define the hepatitis story."


BE PART OF IT
A transformative global health investment
Our funding model is designed to make the most of every dollar you invest. Join us and be part of a community of changemakers committed to ending viral hepatitis.







BE PART OF IT
A transformative global health investment
Our funding model is designed to make the most of every dollar you invest. Join us and be part of a community of changemakers committed to ending viral hepatitis.
Latest News
Photo credits: Emily Jones-Artistry Collective; Max Chang-Visual Impact Agency; Sarah Smith-Imagery Solutions; John Doe-Creative Capture Studios; THF/Claudia Molina.



